HCL/French reference center on AE and PNS

What is a reference center?
A reference center for a rare disease or a group of rare diseases is a set of multidisciplinary hospital skills organized around highly specialized medical teams. A disease is called "rare" when it affects one in 2 000 people, it means that for France, less than 30 000 sick people per pathology.
Reference center purposes
- Disseminate PNS and AE knowledges
- Promote the diagnostic of these diseases
- Reduce the delay between the diagnostic and the patient care
- Improve the quality of the patient care
- Promote research
- Lead university, post-university and extra-university courses
Role and organisation
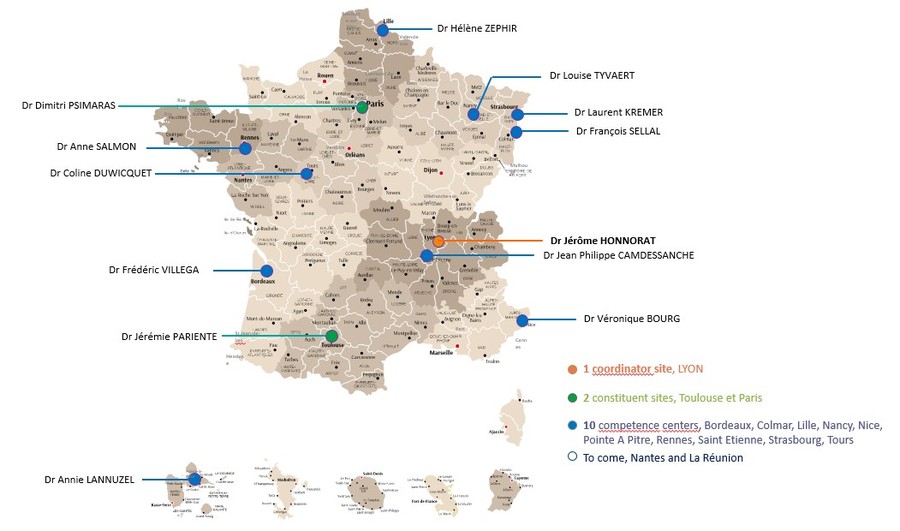
The Reference Center brings together 1 coordinating site (Lyon), 2 constituent sites (Paris and Toulouse) and 10 competence centers (Bordeaux, Colmar, Lille, Nancy, Nice, Pointe A Pitre, Renne, Saint-Etienne, Strasbourg and Tour). The coordinating site manages the network dedicated to SNP and EA, the constituent sites provide complementary expertise, recourse, research or training and the competence centers take care of and monitor patients as close as possible to their home .
The reference center joined the BRAIN TEAM sector in december 2015. This team brings together a group of rare diseases having in common the rare pathologies of the central nervous system in their broadest dimension. Thus, the Brain team combines pathologies with motor or cognitive expression, familial or sporadic in both adults and children.
Reasearch
● Clinical research
Creation and managment of a national database called BRAINDYS, at the origin of many scientifc works which allow the improvment of knowledges on AE and PNS.
The reference center conducted the IasON clinical trial, dedicated to the treament using intravenous immunoglobulins from patients with paraneoplastic neurological syndrome.
● Diagnostic research
Identification of new antibodies, development and provision of diagnostic tests for the nationam medical community and scientific.
● Fundamental research
Development of cellular and animals models in order to understand the mecanisms involved in the neuronal and synaptic dysfunctions characterizing neuropsychiatric diseases and to propose new treatments.
The reference center in figures
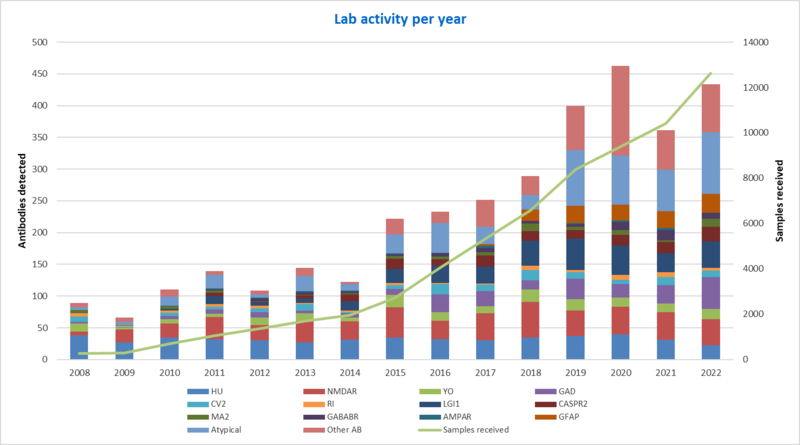
This activity is developed in close collaboration with the CRB of the Lyon neurological hospital "Neurobiotec" and all patients are informed and sign a consent.
The Reference Center's biological collection includes: 2288 sera, 1371 cerebrospinal fluids, 1134 DNA, 441 lymphocytes and 327 tumors and the database now includes more than 5,000 patients.
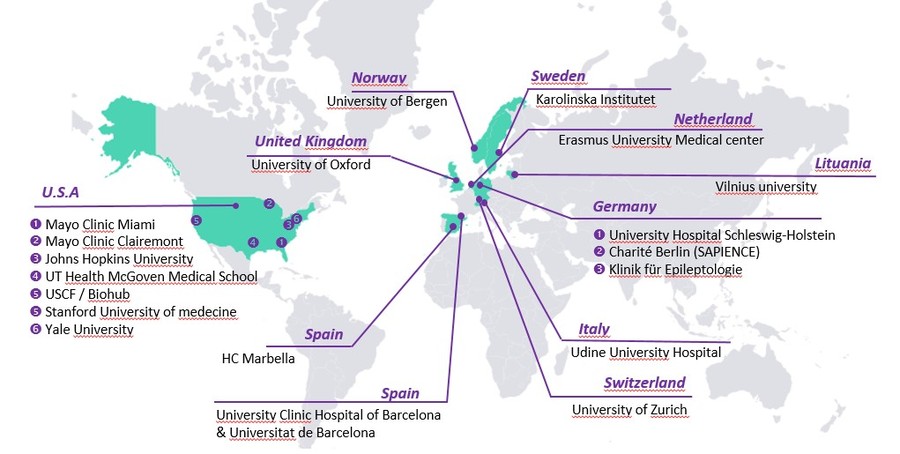
International collaborations
The Center currently has more than 18 international collaborations which have led to the publication of more than 30 scientific articles in high-ranking international journals.