WP1-Prospective collection of patient cohorts with PNS and AE
Leader : J. Honnorat (MD, PhD, Coordinator of the FHU interest, MeLiS/Inserm U1314/UMR CNRS 5284)
Partners: All clinical teams
Databases collecting reliable clinical data and biological samples are mandatory for the identification of new biomarkers in AE and PNS. We already have a collection of such patients with more than 3000 different patients in the database and more than 1300 patients samples in our biobank (CSF, sera, DNA, lymphocytes and tumor tissues) which will be used in WP2,3,4,5. Another mandatory point for the development of future projects is to connect the biobank and collected patient samples with clinical database and to develop real time management of biological samples.
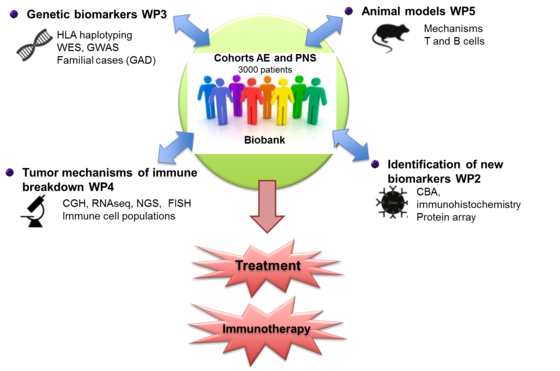
The aim of WP1 consists to standardize, organize and increase our collection of clinico-biological data of patients with different subtypes of AE and PNS as well as to develop a biological collection of specific groups of control patients and to insure quality control of the clinical and biological collections.
Task 1.1: Improvement of patient retrospective cohorts
Objective: Improve database “Braindys” in close relationship with the database of Hospices Civils de Lyon to develop a database summarizing the clinical data of these patients and the available samples in real time to facilitate all the work of WP2, 3 and 4 and the identification of potential new biomarkers.
Task 1.2: Construction of prospective patient cohorts
Objective: Collect clinical data and biological samples of patients with high suspicion of PNS or AE but without identified autoantibodies to build-up a cohort of patients with potential new biomarkers that will be identified in WP2.
Task 1.3: Construction of prospective control cohorts
Objective: Establish control cohorts with well-conserved biological samples. Each cohort will be constituted of the number of patients necessary to validate the usefulness of new identified biomarkers.
Task 1.4: Collection of reference material
Objective: Collect one sample of each patient with a new validated autoantibody in AE or PNS to be used as reference sample.


